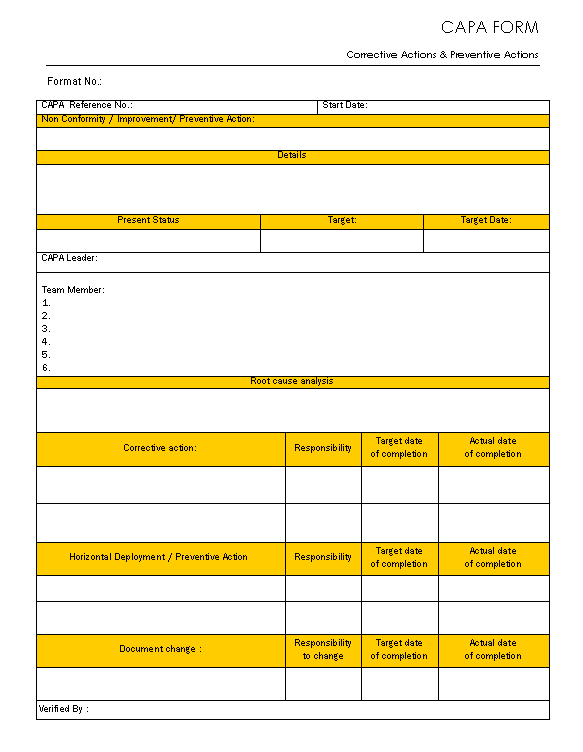
Capa Template
Capa Template - Identify the actions you or others will take to address the root cause, the individual Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. Find 44 templates for creating corrective action plans to deal with and prevent undesirable situations at work. This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. Capa plans must be thorough and well documented. How to fill up the capa format quickly? Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. In your plan, include information that is: Learn what a corrective action plan is, when to use it, and how to write it step by step. Download a free template for sop corrective and preventive action (capa) according to iso 13485:2016. The template includes process steps, participants, inputs,. Corrective action plan templates ensure consistency, clarity, and accountability in addressing and resolving issues across various industries and contexts, such as quality management,. Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. Follow the below steps to fill up the capa format/template quickly; This guide walks businesses through the capa process step by step, ensuring they are prepared to face challenges and raise their standards. Capa plans must be thorough and well documented. It includes a problem statement, action. Download a free template for sop corrective and preventive action (capa) according to iso 13485:2016. Learn what a corrective action plan is, when to use it, and how to write it step by step. Various events may lead to creation of capa. Resolving issues for sustainable solutions: Capa is written to identify a discrepancy or problem in the conduct of. This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. How to fill up the capa format quickly? Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action. This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. Corrective action plan templates ensure consistency, clarity, and accountability in addressing and resolving issues across various industries and contexts, such as quality management,. Capa is written to identify a discrepancy or problem in the conduct of the. How to fill up the capa format quickly? Corrective action plan templates ensure consistency, clarity, and accountability in addressing and resolving issues across various industries and contexts, such as quality management,. This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. This can be used by compliance. It includes a problem statement, action. Learn what a corrective action plan is, when to use it, and how to write it step by step. Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. Capa, or corrective. The main objectives of capa are: Various events may lead to creation of capa. Find 44 templates for creating corrective action plans to deal with and prevent undesirable situations at work. Resolving issues for sustainable solutions: Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified. Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. Learn what a corrective action plan is, when to use it, and how to write it step by step. The template includes process steps, participants, inputs,. The main. Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. Capa plans must be thorough and well documented. Corrective action plan templates ensure consistency, clarity, and accountability in addressing and resolving issues across various industries and contexts, such. Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. Capa, or corrective action and. Corrective action plan templates ensure consistency, clarity, and accountability in addressing and resolving issues across various industries and contexts, such as quality management,. This can be used by compliance officers. This guide walks businesses through the capa process step by step, ensuring they are prepared to face challenges and raise their standards. Capa is written to identify a discrepancy or. Learn what a corrective action plan is, when to use it, and how to write it step by step. It includes a problem statement, action. Identify the actions you or others will take to address the root cause, the individual Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. Follow the below steps to fill up the capa format/template quickly; Download a free template for sop corrective and preventive action (capa) according to iso 13485:2016. Capa plans must be thorough and well documented. Various events may lead to creation of capa. The template includes process steps, participants, inputs,. Capa is written to identify a discrepancy or problem in the conduct of the clinical research study, note the root cause of the identified problem, identify the corrective action taken to prevent. Corrective action plan templates ensure consistency, clarity, and accountability in addressing and resolving issues across various industries and contexts, such as quality management,. The main objectives of capa are: This can be used by compliance officers. In your plan, include information that is: This document is a capa (corrective and preventive action) template used to document issues, analyze root causes, and implement corrective and preventive actions. How to fill up the capa format quickly?Capa Report Template
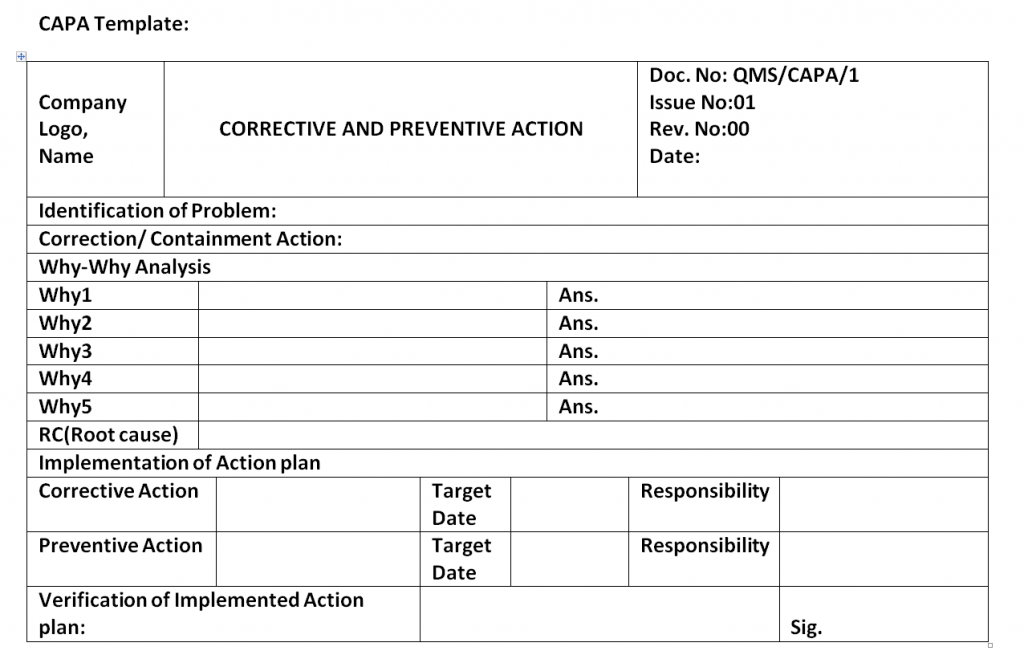
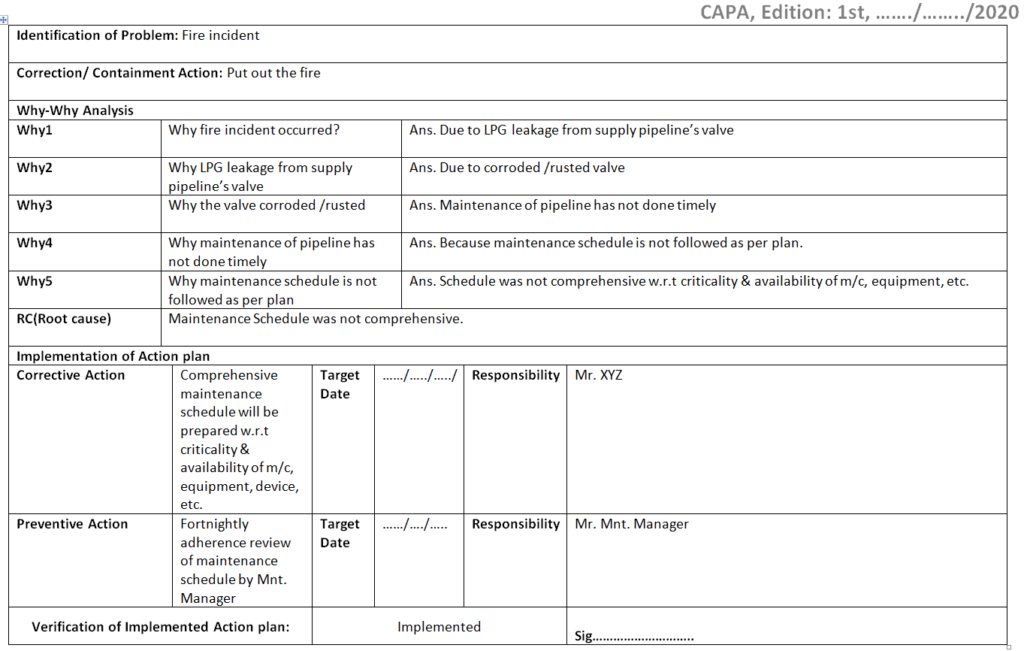
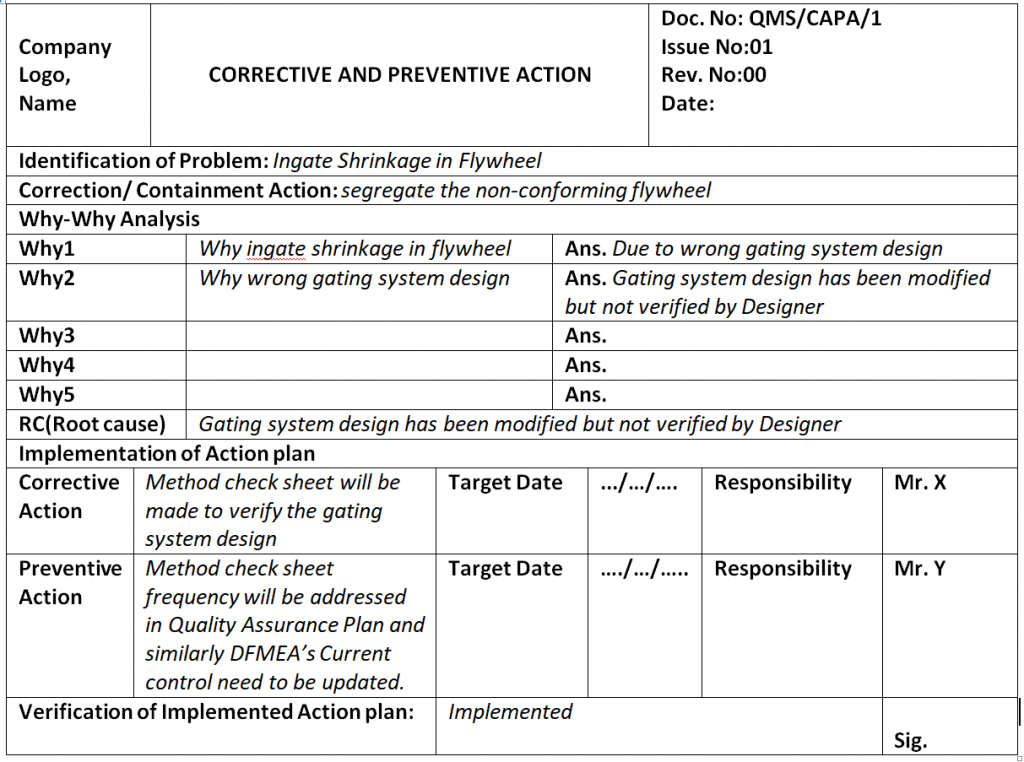
Corrective and Preventive Action Format CAPA with Example
Capa Plan Template
Capa Form Template Free Free Sample, Example & Format Templates
Corrective and Preventive Action Format CAPA with Example
Sample Capa Form
Corrective Action Preventive Action Template
Capa Template Printable Word Searches
CAPA form Corrective action and preventive action
Capa Plan Template
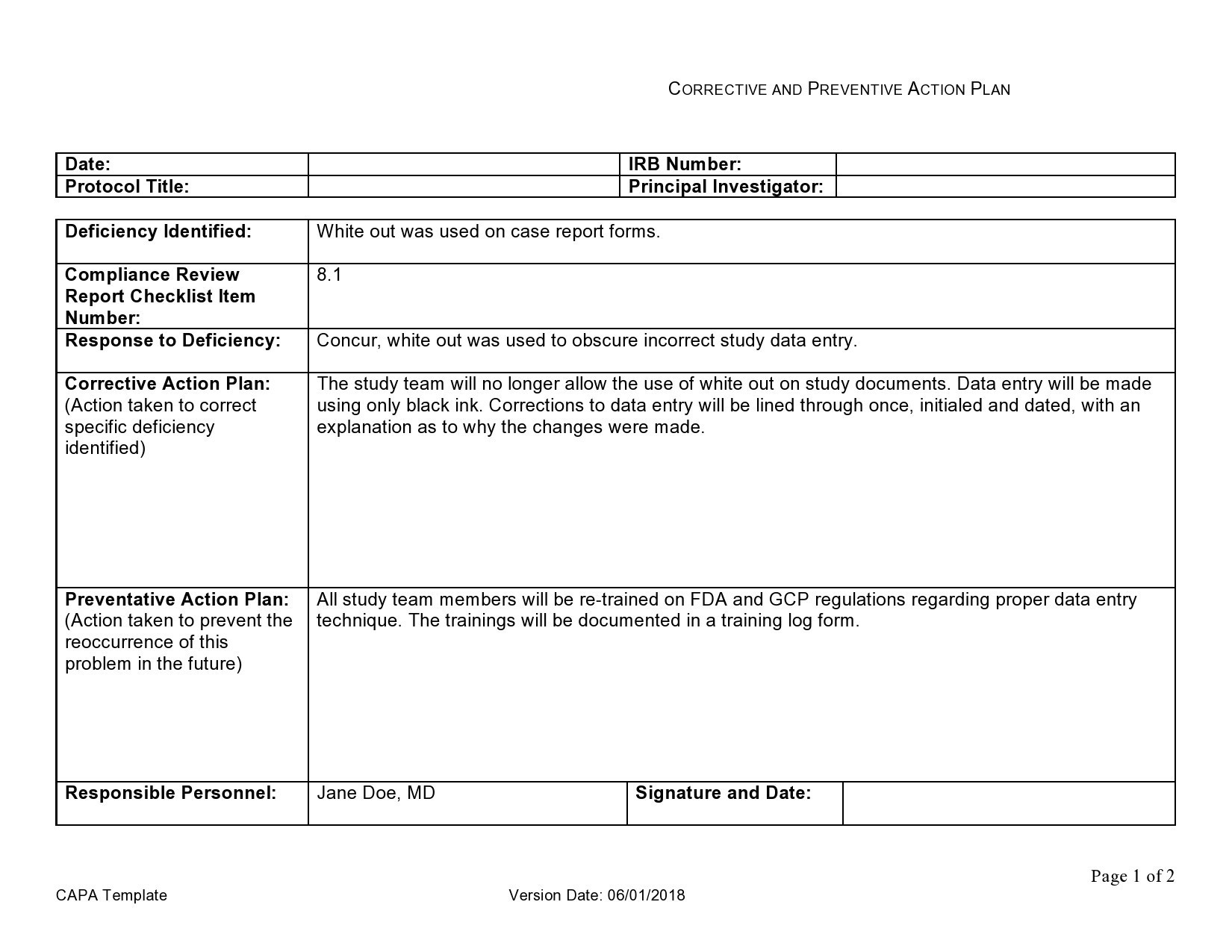
Capa, Or Corrective Action And Preventive Action, Can Provide A Structure For Finding The Root Cause Of Problems, Solving Those Problems, Documenting The Conditions And.
This Document Is A Capa (Corrective And Preventive Action) Template Used To Document Issues, Analyze Root Causes, And Implement Corrective And Preventive Actions.
Resolving Issues For Sustainable Solutions:
This Guide Walks Businesses Through The Capa Process Step By Step, Ensuring They Are Prepared To Face Challenges And Raise Their Standards.
Related Post: