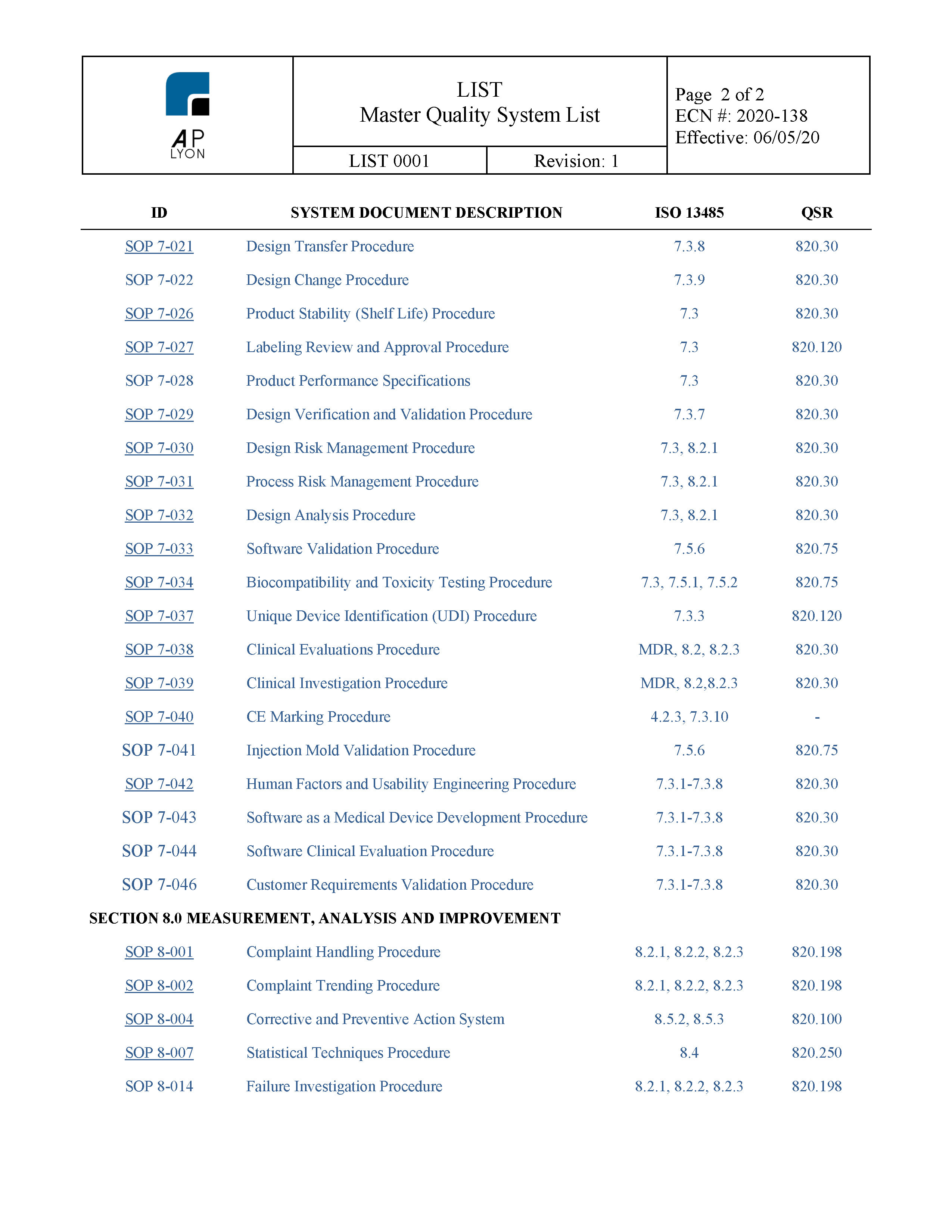
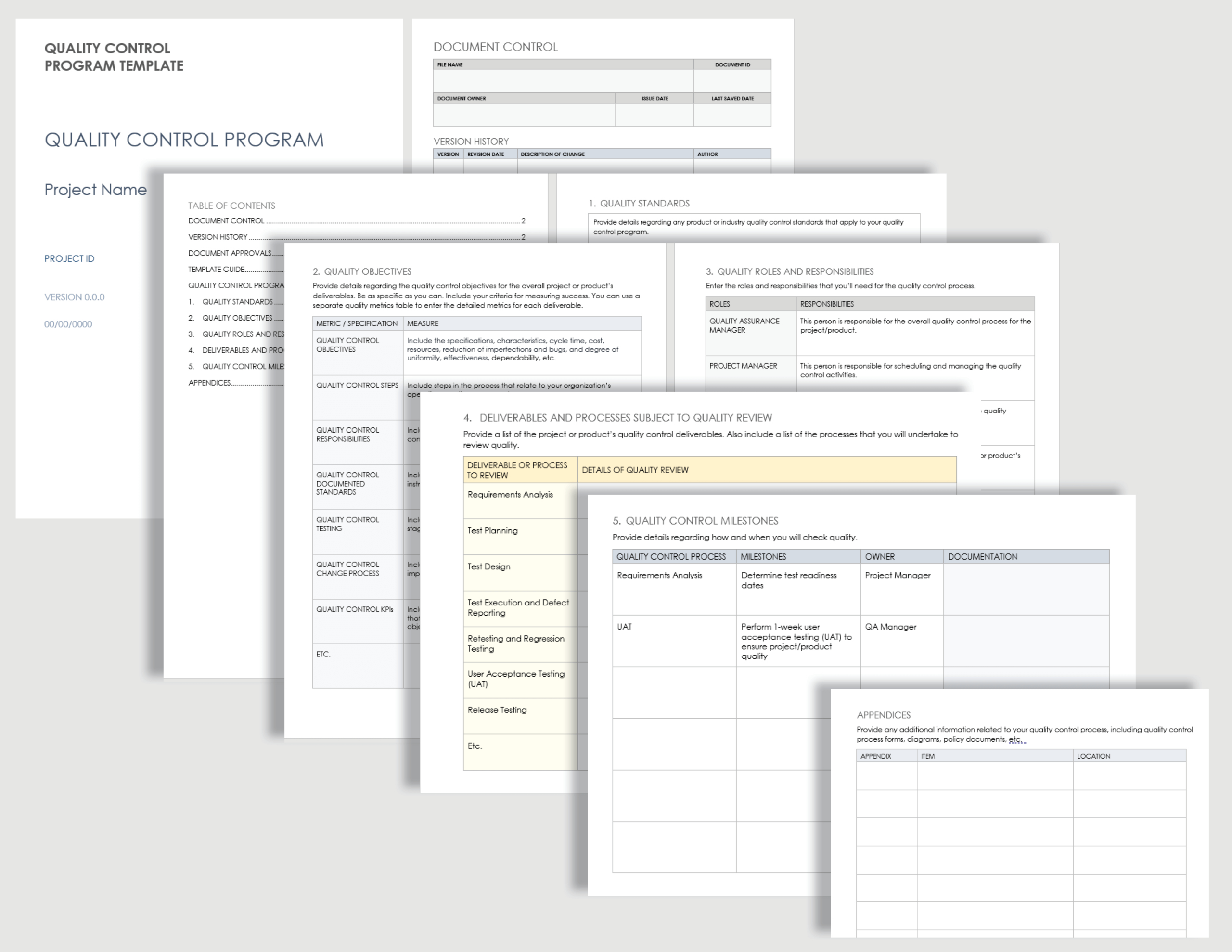
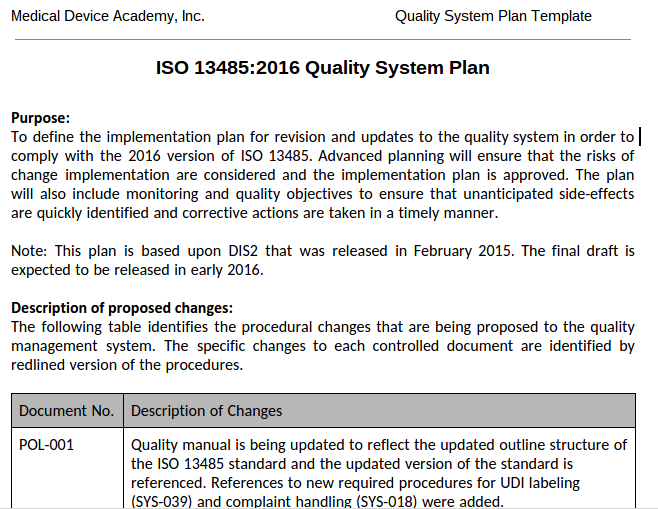
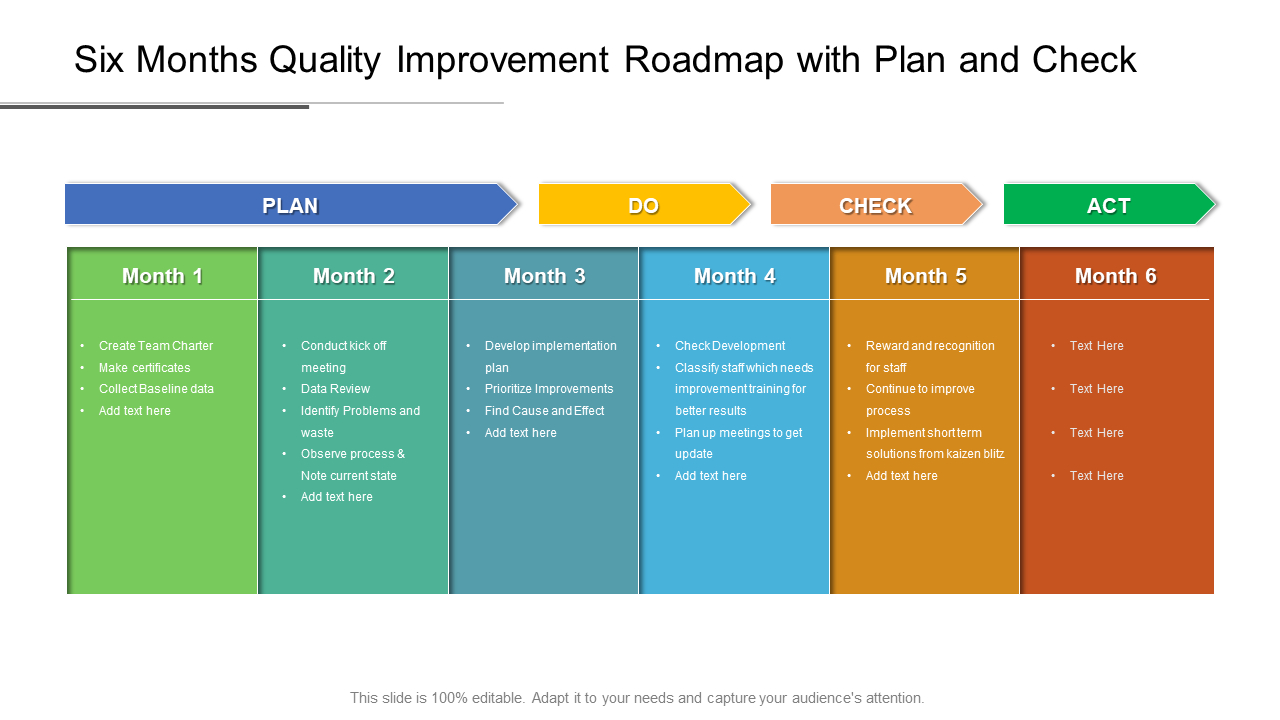
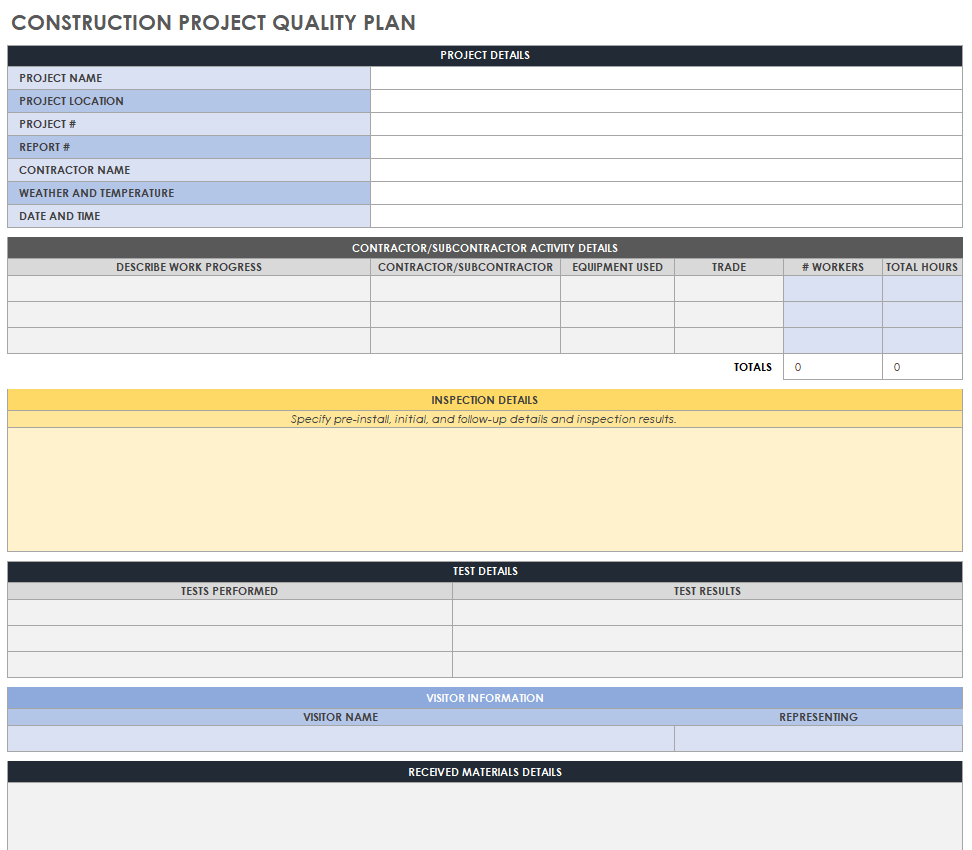
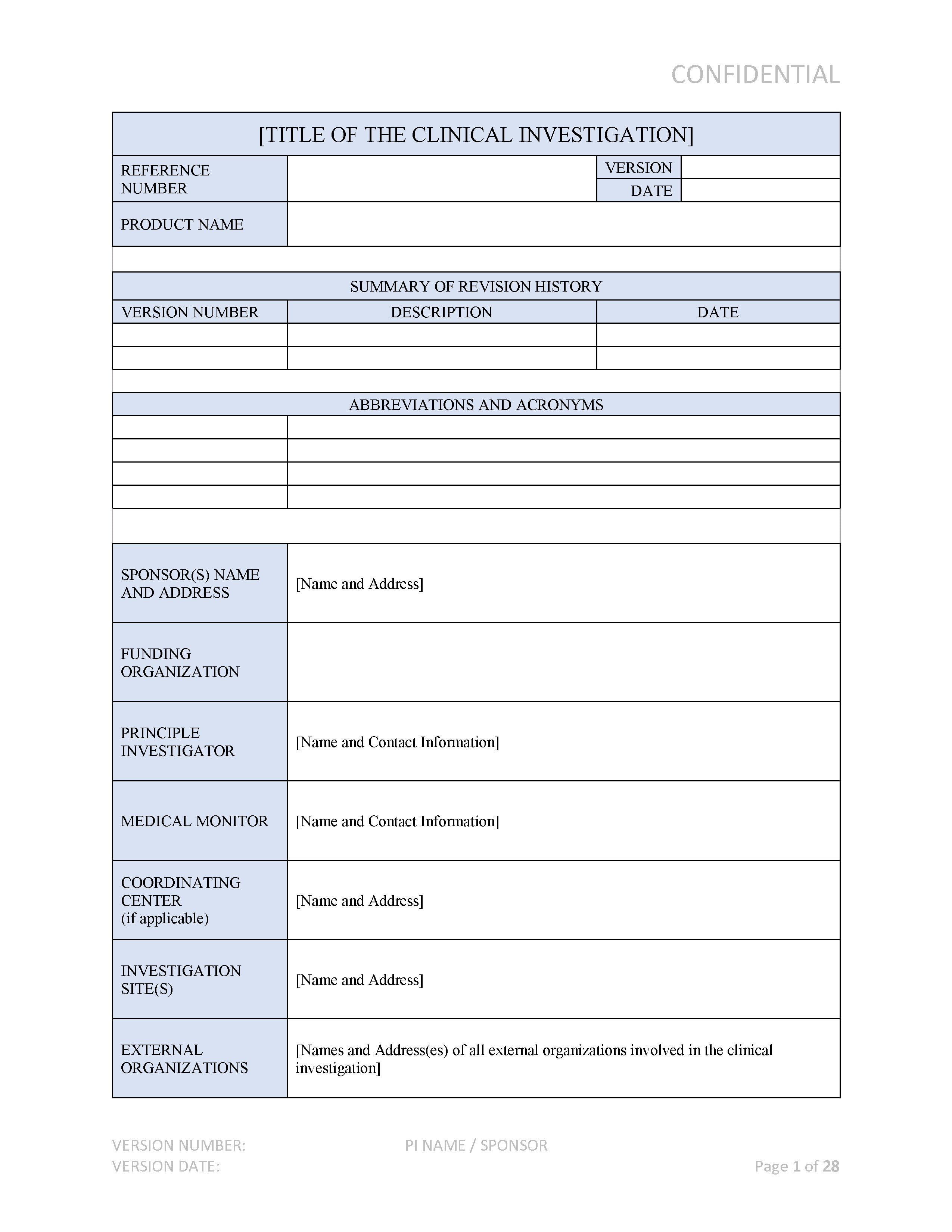
Example Of Medical Device Quality Plan Template
Example Of Medical Device Quality Plan Template - Implementation and training products for medical device quality management systems (qms) according to the iso 13485 standard. Plan, do check, and act (pdca) is the mantra of the deming disciples, but does anyone know what should be in your quality system plan template. Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. Additionally, we’ve also got templates for the mdr clinical. Improve your medical device quality management with our comprehensive quality plan template! This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. The iso 13485 is the standard for quality management in the medical device industry. Each of the quality plan types described in this article has distinct requirements for successful implementation. This article provides a blueprint you can use to create a quality management plan that enables your company to achieve quality goals, redirects you if you go off course, and. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured. The medqdoc configuration is built. Each of the quality plan types described in this article has distinct requirements for successful implementation. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory requirements. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. Implementation and training products for medical device quality management systems (qms) according to the iso 13485 standard. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured. This article provides a blueprint you can use to create a quality management plan that enables your company to achieve quality goals, redirects you if you go off course, and. Additionally, we’ve also got templates for the mdr clinical. Plan, do check, and act (pdca) is the mantra of the deming disciples, but does anyone know what should be in your quality system plan template. Here are all our posts on this standard, and also all questions our consulting clients. We will focus on the pdp quality plan in. Additionally, we’ve also got templates for the mdr clinical. Improve your medical device quality management with our comprehensive quality plan template! Plan, do check, and act (pdca) is the mantra of the deming disciples, but does anyone know what should be in your quality system plan template. Complete iso 13485 and. The iso 13485 is the standard for quality management in the medical device industry. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. Here are all our posts on this standard, and also all questions our consulting clients. Complete. This article provides a blueprint you can use to create a quality management plan that enables your company to achieve quality goals, redirects you if you go off course, and. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory requirements. Complete iso 13485 and fda qsr compliant quality system. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. Each of the quality plan types described in this article has distinct requirements for successful implementation. Ensure compliance, streamline processes, and achieve outstanding product quality with ease. Complete iso 13485. The iso 13485 is the standard for quality management in the medical device industry. Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. Additionally, we’ve. Plan, do check, and act (pdca) is the mantra of the deming disciples, but does anyone know what should be in your quality system plan template. The iso 13485 is the standard for quality management in the medical device industry. Improve your medical device quality management with our comprehensive quality plan template! This article provides a blueprint you can use. Plan, do check, and act (pdca) is the mantra of the deming disciples, but does anyone know what should be in your quality system plan template. The iso 13485 is the standard for quality management in the medical device industry. Implementation and training products for medical device quality management systems (qms) according to the iso 13485 standard. Each manufacturer shall. Implementation and training products for medical device quality management systems (qms) according to the iso 13485 standard. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. Each of the quality plan types described in this article has distinct requirements. Additionally, we’ve also got templates for the mdr clinical. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured. We will focus on the pdp quality plan in. This medical devices development plan describes in detail all essential steps to be considered prior to start the development. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured. The iso 13485 is the standard for quality management in the medical device industry. Improve your medical device quality management with our comprehensive quality plan template! Implementation and training products for medical device quality management systems (qms). In this article, we will cover the iso 13485 and fda requirements for a quality policy, and provide examples of quality policies from various medical device companies. The medqdoc configuration is built. Complete iso 13485 and fda qsr compliant quality system templates for medical device businesses. This medical devices development plan describes in detail all essential steps to be considered prior to start the development of medical devices and is in alignment with current fda and. Implementation and training products for medical device quality management systems (qms) according to the iso 13485 standard. Ensure compliance, streamline processes, and achieve outstanding product quality with ease. Medqdoc provides 28 templates to support you in compiling the correct technical documentation for your medical device, to meet regulatory requirements. Improve your medical device quality management with our comprehensive quality plan template! The iso 13485 is the standard for quality management in the medical device industry. This article provides a blueprint you can use to create a quality management plan that enables your company to achieve quality goals, redirects you if you go off course, and. Additionally, we’ve also got templates for the mdr clinical. Each manufacturer shall establish a quality plan which defines the quality practices, resources, and activities relevant to devices that are designed and manufactured.Medical Device Quality Plan Template
Sample Of Medical Device Quality Plan Template
Medical Device Quality Plan Template Sample Template Samples
Medical Device Design And Development Plan QMDocs Quality Management
Medical device quality management system template 8 powerful options
How to write a quality system plan template (free download)
Medical Device Quality Plan Template Sample Template Samples
Medical Device Quality Plan Template Sample Template Samples
Medical Device Development Plan Template in Word, Pages, Google Docs
Medical Device Quality Plan Template
Here Are All Our Posts On This Standard, And Also All Questions Our Consulting Clients.
Plan, Do Check, And Act (Pdca) Is The Mantra Of The Deming Disciples, But Does Anyone Know What Should Be In Your Quality System Plan Template.
We Will Focus On The Pdp Quality Plan In.
Each Of The Quality Plan Types Described In This Article Has Distinct Requirements For Successful Implementation.
Related Post: