Fdp Subaward Template
Fdp Subaward Template - While only the changes to the cost reimbursement subaward will be. It is the period for. Outline the major changes to the fdp subaward template from the 2019 version to the 2020 version. It covers the required attachments, certifications, and. Find answers to common questions about the fdp subaward templates and samples for cost reimbursement, fixed amount, foreign, and clinical research subawards. The new templates (version dated oct 2019) are for use with all new subawards issued under grants and cooperative agreements awarded or amended after december 26, 2014 that. Below are some fdp resources to assist your organization with your subrecipient processes. A pdf document that explains how to use the fdp template for issuing subawards to subrecipients under federal awards. The statement of work and budget for this subaward are as shown in. A pdf template for a cost reimbursable subaward between a prime recipient and a subrecipient, with terms and conditions, certifications and assurances. It covers the required attachments, certifications, and. Find answers to common questions about the fdp subaward templates and samples for cost reimbursement, fixed amount, foreign, and clinical research subawards. If applying its own financial conflicts of interest policy, by execution of this subaward agreement, subrecipient institution certifies that its policy complies with the requirements of the relevant. The statement of work and budget for this subaward are as shown in. Learn about the fdp subawards subcommittee's projects,. A pdf template for a cost reimbursable subaward between a prime recipient and a subrecipient, with terms and conditions, certifications and assurances. Fdp subawards templates working group maintains the accuracy of federal regulations and terms & conditions to be flowed down to subrecipients, as well as lists of requested changes. By signing the subaward agreement, the authorized official of collaborator certifies, to the best of his/her knowledge and belief, that: It is intended for organizations that are submitting a proposal to the u.s. Va’s office of general counsel strongly recommends nonprofit corporations work with the specialty team advising research before entering subaward/subcontract. If applying its own financial conflicts of interest policy, by execution of this subaward agreement, subrecipient institution certifies that its policy complies with the requirements of the relevant. Learn about the fdp subawards subcommittee's projects,. It is intended for organizations that are submitting a proposal to the u.s. The subaward module in kuali research has the ability to pull entered. Learn how to use the updated templates,. While only the changes to the cost reimbursement subaward will be. Find answers to common questions about the fdp subaward templates and samples for cost reimbursement, fixed amount, foreign, and clinical research subawards. The new templates (version dated oct 2019) are for use with all new subawards issued under grants and cooperative agreements. A pdf template for a cost reimbursable subaward between a prime recipient and a subrecipient, with terms and conditions, certifications and assurances. If applying its own financial conflicts of interest policy, by execution of this subaward agreement, subrecipient institution certifies that its policy complies with the requirements of the relevant. Find answers to common questions about the fdp subaward templates. The new templates (version dated oct 2019) are for use with all new subawards issued under grants and cooperative agreements awarded or amended after december 26, 2014 that. While only the changes to the cost reimbursement subaward will be. On the ug fdp face page and amendment templates, the period of performance meets the requirements of subaward period of performance. Assess your subrecipient management practices. Outline the major changes to the fdp subaward template from the 2019 version to the 2020 version. It covers the required attachments, certifications, and. Va’s office of general counsel strongly recommends nonprofit corporations work with the specialty team advising research before entering subaward/subcontract. A pdf template for a cost reimbursable subaward between a prime recipient. Outline the major changes to the fdp subaward template from the 2019 version to the 2020 version. Learn how to complete the fdp subaward amendment templates and understand the changes from the prior versions. Learn how to use the fdp subcontract template and sample for federal assistance awards (grants and cooperative agreements) and federal contracts. Va’s office of general counsel. National science foundation or have received an nsf. Learn about the fdp subawards subcommittee's projects,. Learn how to use the fdp subcontract template and sample for federal assistance awards (grants and cooperative agreements) and federal contracts. Assess your subrecipient management practices. A pdf document that explains how to use the fdp template for issuing subawards to subrecipients under federal awards. While only the changes to the cost reimbursement subaward will be. The new templates (version dated oct 2019) are for use with all new subawards issued under grants and cooperative agreements awarded or amended after december 26, 2014 that. Find instructions, field numbers, and examples for bilateral and. Pte hereby awards a cost reimbursable subaward, (as determined by 2 cfr. Outline the major changes to the fdp subaward template from the 2019 version to the 2020 version. Va’s office of general counsel strongly recommends nonprofit corporations work with the specialty team advising research before entering subaward/subcontract. It is the period for. National science foundation or have received an nsf. Pte hereby awards a cost reimbursable subaward, (as determined by 2. The new templates (version dated oct 2019) are for use with all new subawards issued under grants and cooperative agreements awarded or amended after december 26, 2014 that. Find answers to common questions about the fdp subaward templates and samples for cost reimbursement, fixed amount, foreign, and clinical research subawards. Below are some fdp resources to assist your organization with. Va’s office of general counsel strongly recommends nonprofit corporations work with the specialty team advising research before entering subaward/subcontract. Assess your subrecipient management practices. By signing the subaward agreement, the authorized official of collaborator certifies, to the best of his/her knowledge and belief, that: Find guidance, templates and resources for issuing and managing subawards under federally funded grants and agreements. National science foundation or have received an nsf. Outline the major changes to the fdp subaward template from the 2019 version to the 2020 version. The subaward module in kuali research has the ability to pull entered information and populate fdp boilerplate forms directly pulled from fdp to print and use when issuing outgoing. The statement of work and budget for this subaward are as shown in. The statement of work and budget for this subaward are as shown in attachment 5. Find answers to common questions about the fdp subaward templates and samples for cost reimbursement, fixed amount, foreign, and clinical research subawards. If applying its own financial conflicts of interest policy, by execution of this subaward agreement, subrecipient institution certifies that its policy complies with the requirements of the relevant. The new templates (version dated oct 2019) are for use with all new subawards issued under grants and cooperative agreements awarded or amended after december 26, 2014 that. Pte hereby awards a cost reimbursable subaward, (as determined by 2 cfr 200.331), to subrecipient. Learn about the fdp subawards subcommittee's projects,. Below are some fdp resources to assist your organization with your subrecipient processes. While only the changes to the cost reimbursement subaward will be.Fdp Subaward Template
Fdp Subaward Template
Guidance for Using the FDP Subaward Templates Doc Template pdfFiller
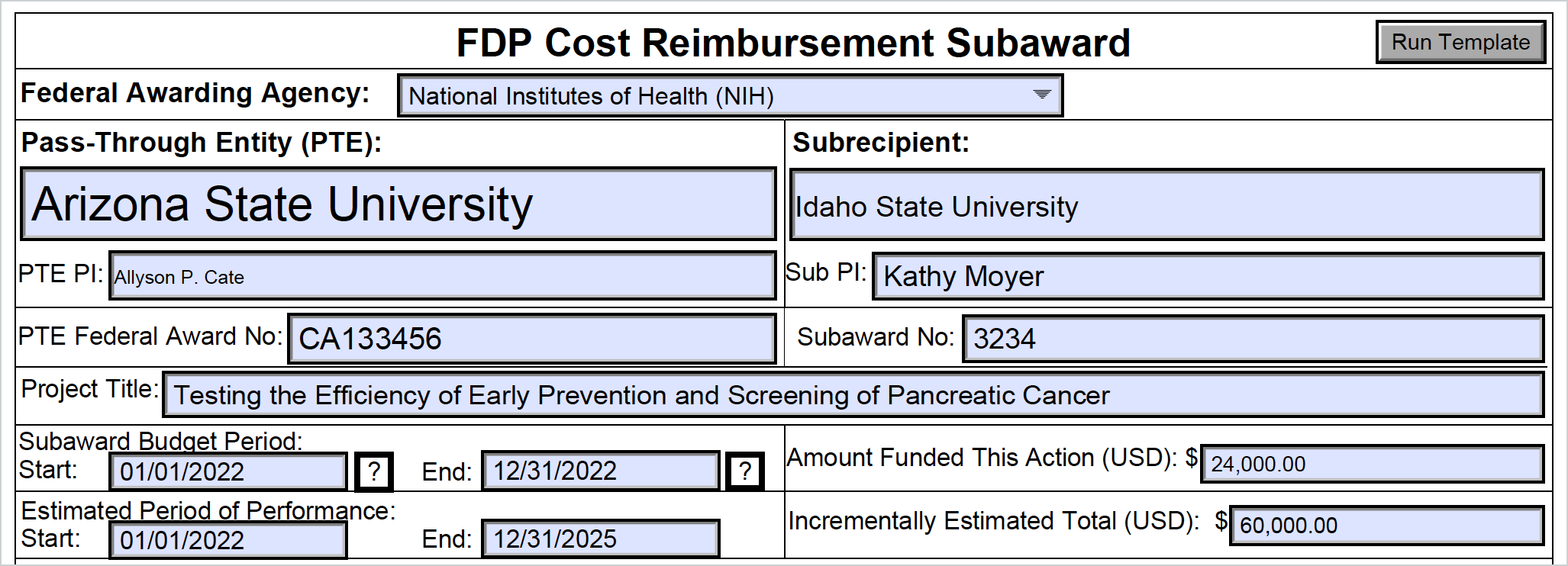
Fdp Subaward Agreement Templates Master Template
Fdp Subaward Template
Fdp subaward template Fill out & sign online DocHub
Fdp Template Fill Online, Printable, Fillable, Blank pdfFiller
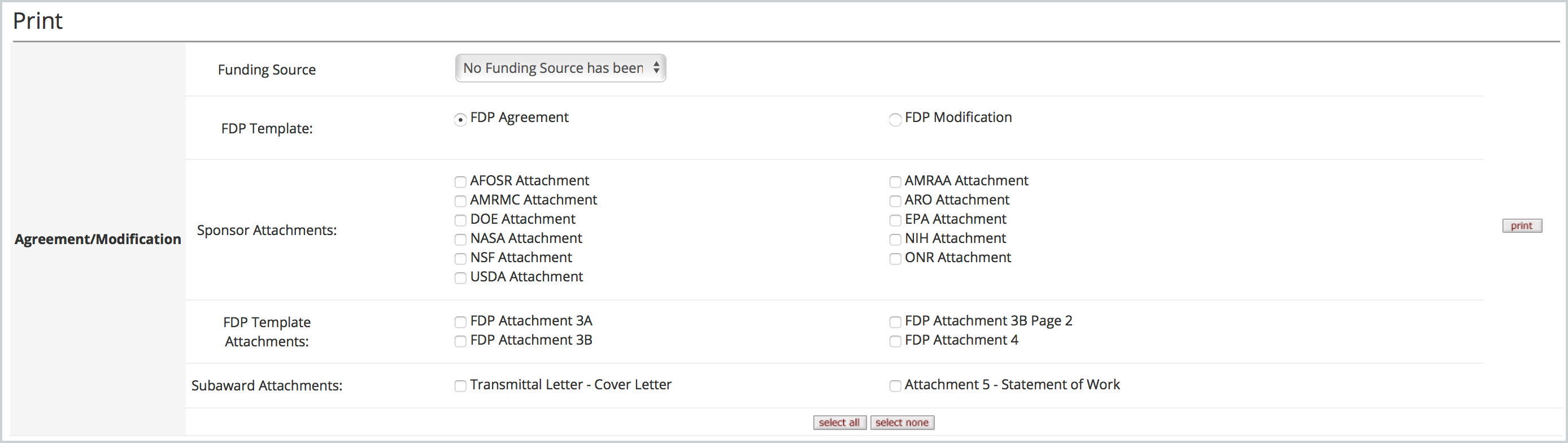
Subaward Template Information & FDP Form Printing Kuali Research
Subaward FDP Forms Kuali Research
Fdp Agreement Template Master Template
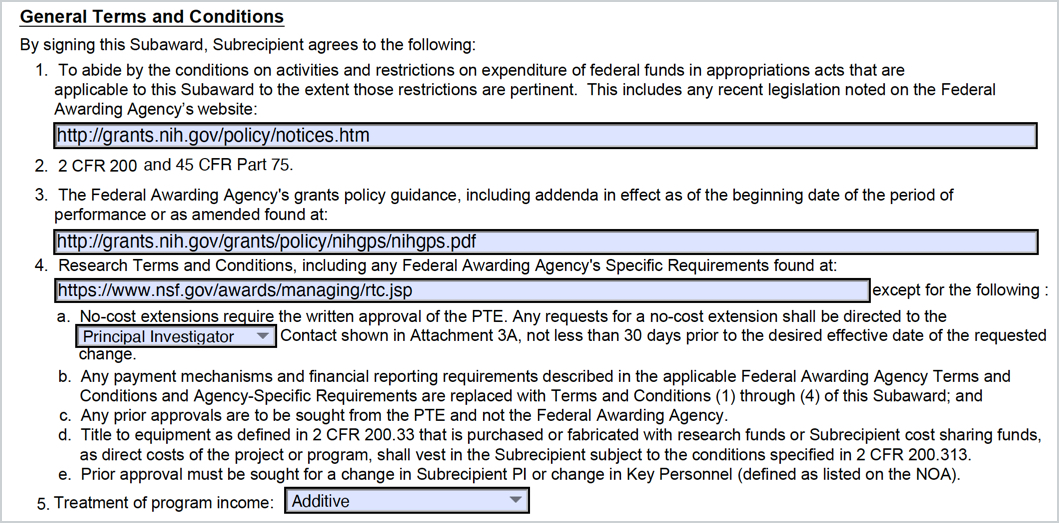
It Covers The Required Attachments, Certifications, And.
Pte Hereby Awards A Fixed Price Subaward, As Described Above, To Subrecipient.
Find Guidance And Templates For Issuing Subawards Under The Uniform Guidance (Ug) By The Federal Demonstration Partnership (Fdp).
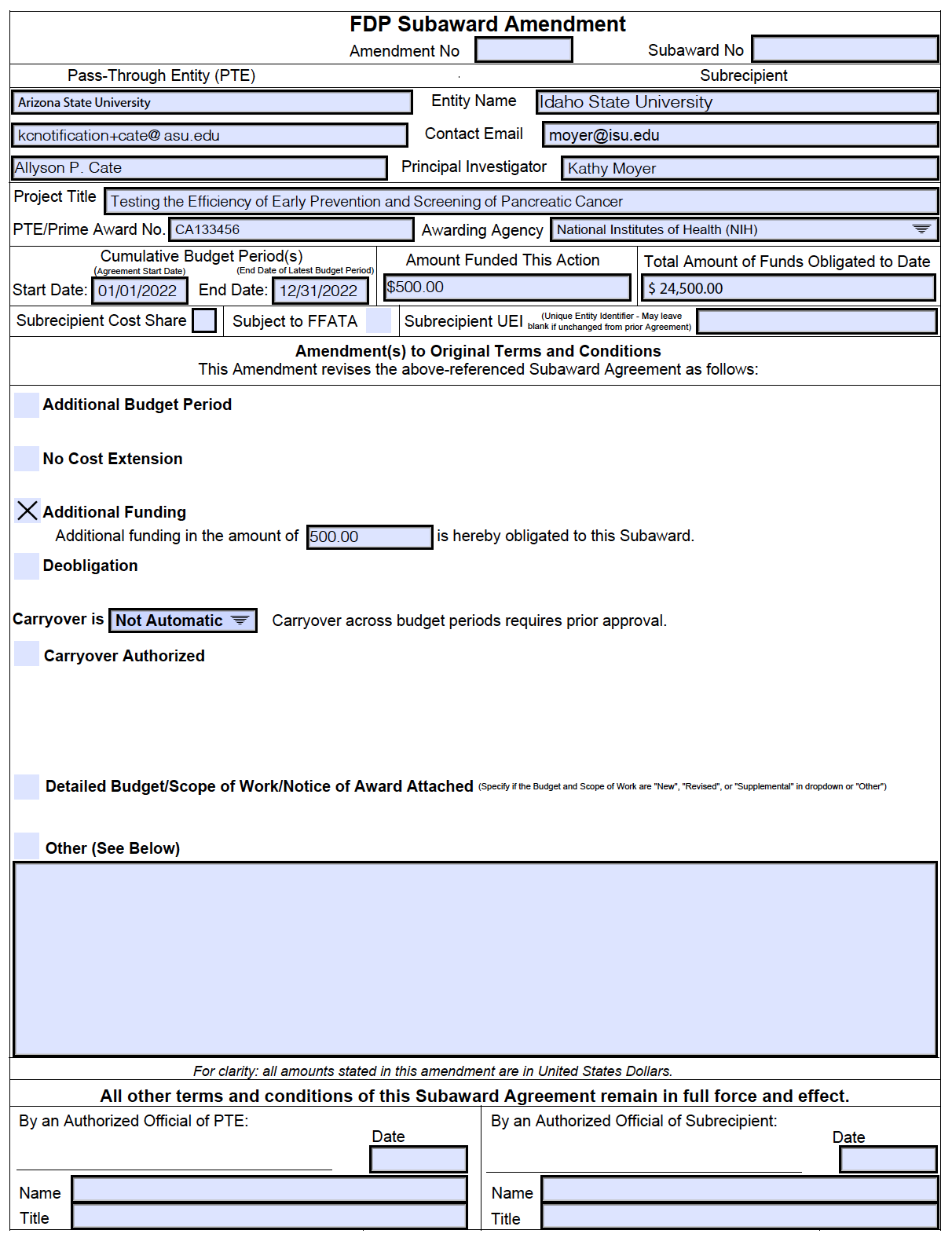
Learn How To Complete The Fdp Subaward Amendment Templates And Understand The Changes From The Prior Versions.
Related Post: