Post Market Surveillance Plan Template
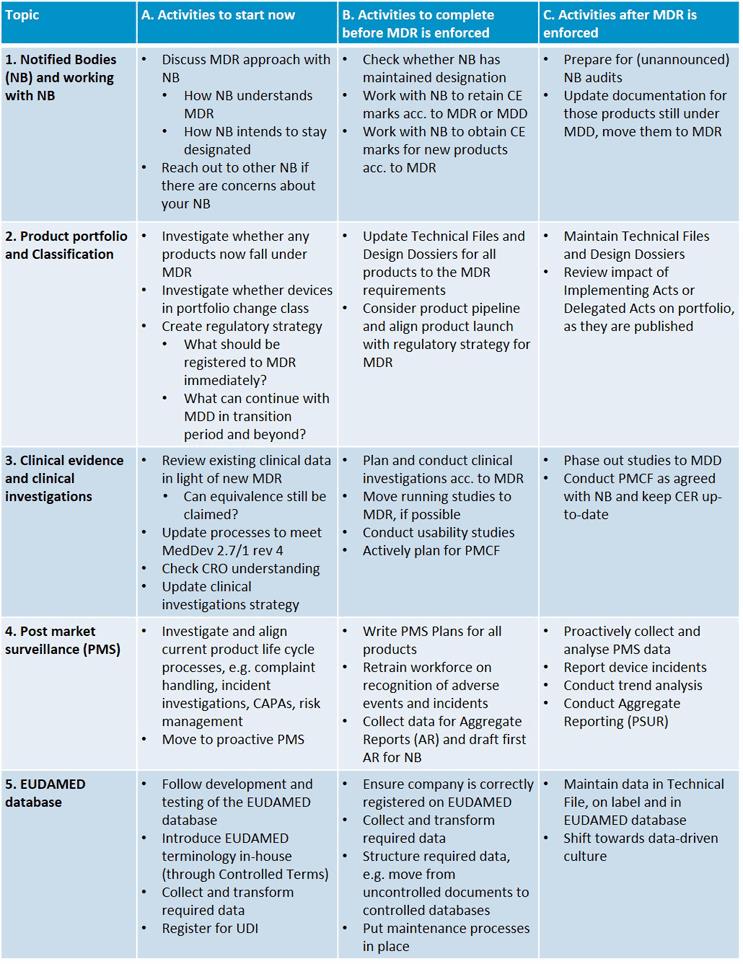
Post Market Surveillance Plan Template - The pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered and. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. This outline was derived from the requirements set. Post market surveillance plan psur document template citemed’s proposed psur template comprises the following detailed sections. The pms plan covers basically three activities. The pms plan forces you to document and evaluate all. It ensures the proactive collection of new safety and performance information, which is then. A medical device post market surveillance plan (pms plan) means all activities carried out by manufacturers in cooperation with other economic operators to institute and. Take a look into our pms plan template. It is impossible to simply ‘template’ the entirety that is post market surveillance; A medical device post market surveillance plan (pms plan) means all activities carried out by manufacturers in cooperation with other economic operators to institute and. It ensures the proactive collection of new safety and performance information, which is then. This outline was derived from the requirements set. Post market surveillance plan psur document template citemed’s proposed psur template comprises the following detailed sections. The pms plan covers basically three activities. The template outlines the content, process and frequency of. The pms plan forces you to document and evaluate all. Download a free template and access. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. Take a look into our pms plan template. On the one hand, they have to comply with the. Information regarding similar medical devices and technologies on the. It is impossible to simply ‘template’ the entirety that is post market surveillance; The pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered and.. Download a free template and access. A medical device post market surveillance plan (pms plan) means all activities carried out by manufacturers in cooperation with other economic operators to institute and. Information regarding similar medical devices and technologies on the. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. Post market surveillance plan psur. Post market surveillance plan psur document template citemed’s proposed psur template comprises the following detailed sections. Download a free template and access. On the one hand, they have to comply with the. This outline was derived from the requirements set. The pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member,. The pms plan covers basically three activities. It is impossible to simply ‘template’ the entirety that is post market surveillance; Download a free template and access. It ensures the proactive collection of new safety and performance information, which is then. Information regarding similar medical devices and technologies on the. A medical device post market surveillance plan (pms plan) means all activities carried out by manufacturers in cooperation with other economic operators to institute and. Download a free template and access. This outline was derived from the requirements set. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. Post market surveillance plan psur document. Post market surveillance plan psur document template citemed’s proposed psur template comprises the following detailed sections. Information regarding similar medical devices and technologies on the. The pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered and. On the one hand, they have to. A medical device post market surveillance plan (pms plan) means all activities carried out by manufacturers in cooperation with other economic operators to institute and. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. Take a look into our pms plan template. The pms plan forces you to document and evaluate all. The pms. The pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered and. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. On the one hand, they have to comply with the. Post market surveillance plan psur document template. It ensures the proactive collection of new safety and performance information, which is then. The template outlines the content, process and frequency of. Information regarding similar medical devices and technologies on the. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. It is impossible to simply ‘template’ the entirety that is post market surveillance; However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. It is impossible to simply ‘template’ the entirety that is post market surveillance; A medical device post market surveillance plan (pms plan) means all activities carried out by manufacturers in cooperation with other economic operators to institute and. The template outlines the content, process and. A medical device post market surveillance plan (pms plan) means all activities carried out by manufacturers in cooperation with other economic operators to institute and. It ensures the proactive collection of new safety and performance information, which is then. The template outlines the content, process and frequency of. The pms plan forces you to document and evaluate all. Download a free template and access. It is impossible to simply ‘template’ the entirety that is post market surveillance; On the one hand, they have to comply with the. This outline was derived from the requirements set. However, in the reactive forms (pmcf, psurs), we can help guide you by creating reusable. The pms plan covers basically three activities. The pms template provides a structured approach to setting up a pms process, defining the responsibilities of each team member, and outlining how feedback will be gathered and.Mdr Post Market Surveillance Plan Template
EU postmarket surveillance plans for medical devices Pane 2019
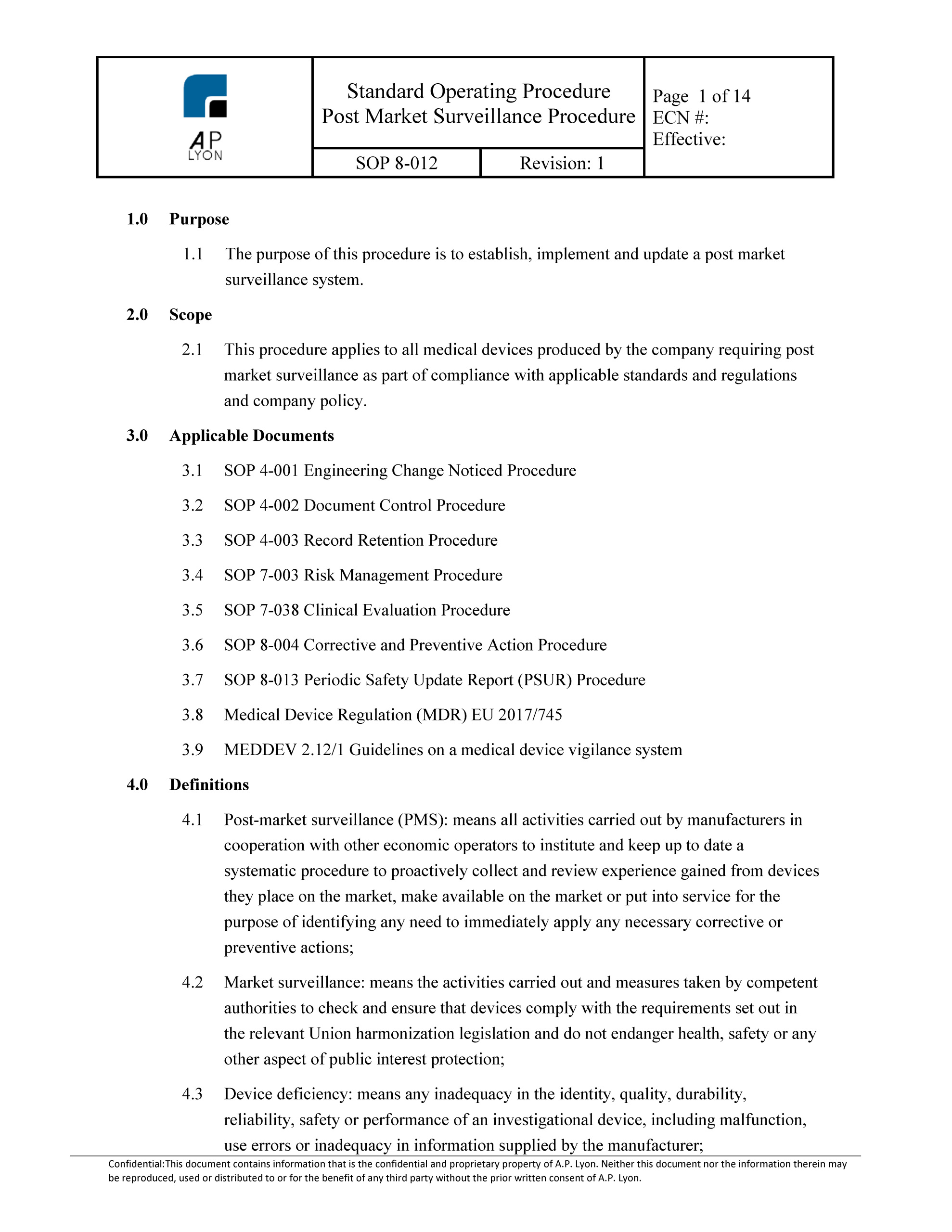
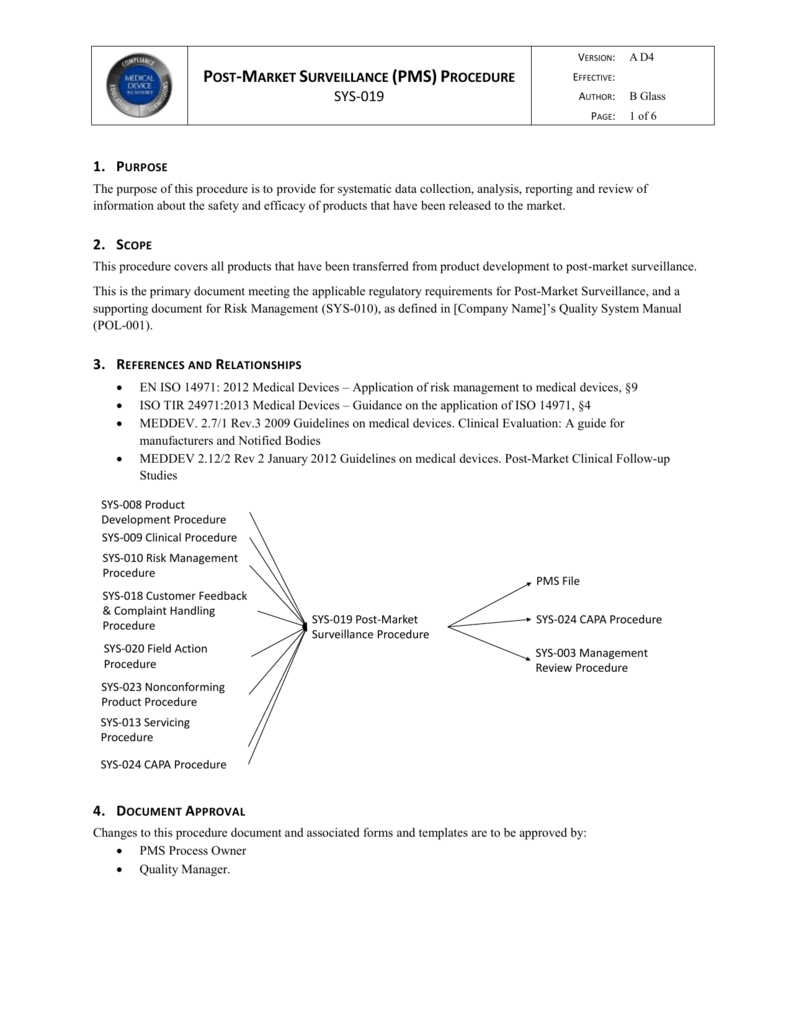
Post Market Surveillance Procedure
EU postmarket surveillance plans for medical devices Pane 2019
EU postmarket surveillance plans for medical devices Pane 2019
Post Market Surveillance Procedure
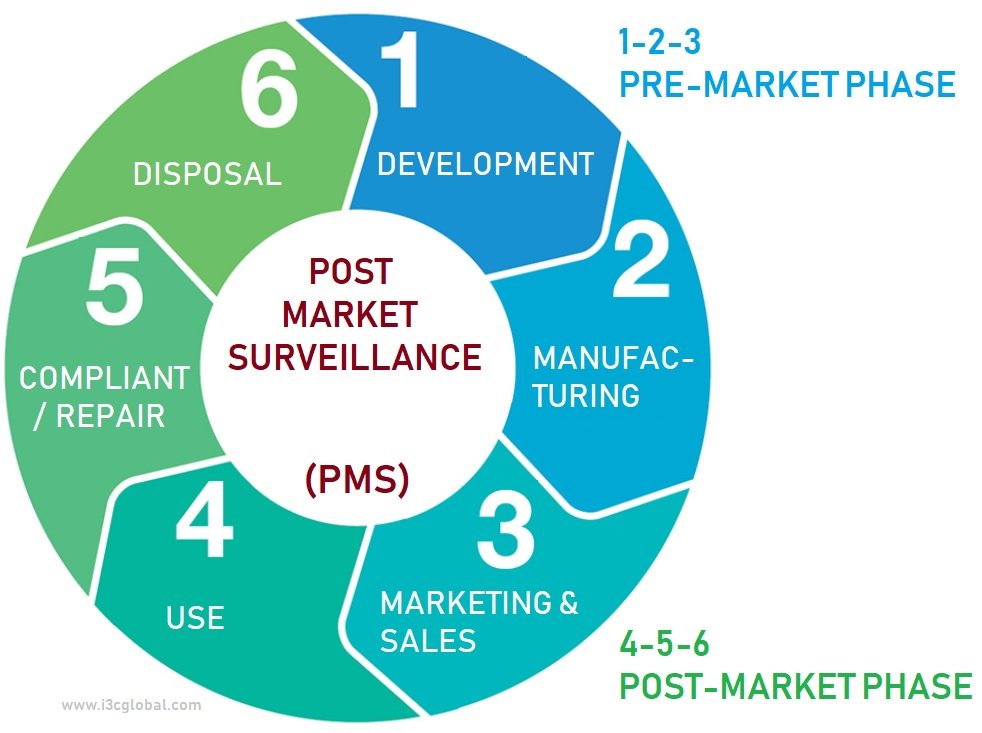
Postmarket surveillance is in itself a monitoring and measuring
Post Market Surveillance Plan PMS Plan Template
Post Market Surveillance Plan PMS Plan Template
Post Market Surveillance Plan Template prntbl.concejomunicipaldechinu
Information Regarding Similar Medical Devices And Technologies On The.
Take A Look Into Our Pms Plan Template.
Post Market Surveillance Plan Psur Document Template Citemed’s Proposed Psur Template Comprises The Following Detailed Sections.
Related Post: