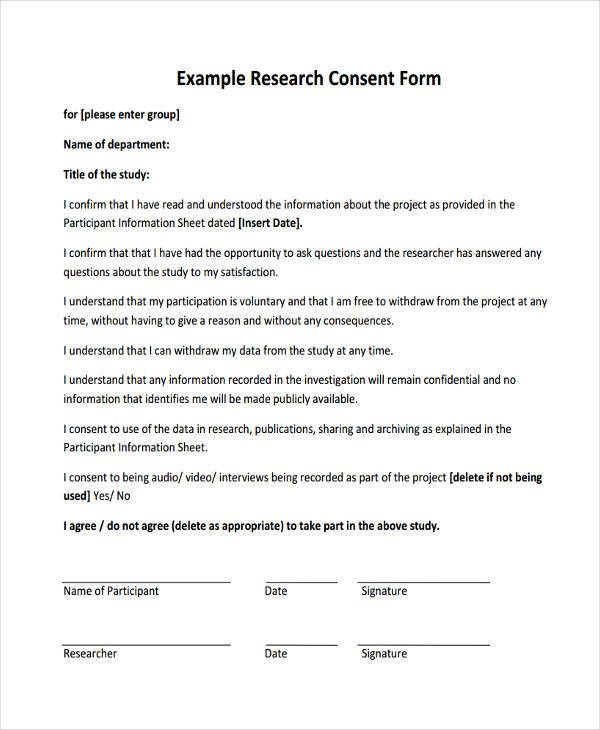
Research Consent Form Template
Research Consent Form Template - This consent should be used to obtain permission from subjects or the parent(s) of subjects (if minors will be. Learn about the changes to the regulations for informed consent and the irb submission. Download the forms in doc format. It is important that principal. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). There are other webpages devoted to providing guidance for writing readable,. The templates on this page are intended to help investigators construct documents that are as short as possible and written in plain language. The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. Informed consent and hipaa authorization form template v01nov2024 docx: The consent/assent form should be in a language that is understandable to someone without a scientific background. Find consent form templates and guidance for different types of research projects. Oprs provides consent document templates as a resource tool for researchers. Learn about the changes to the regulations for informed consent and the irb submission. Learn how to use the new plain. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): The consent/assent form should be in a language that is understandable to someone without a scientific background. This template should be used as the consent document guide for all new research studies, including parental. This resource provides points to consider and sample language for informed consent documents of research studies which plan to store and share data and/ or biospecimens for. Investigators are required to use the latest versions of the informed consent form templates, which have been updated to comply with the 2019. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. Learn about the changes to the regulations for informed consent and the irb submission. Use these templates to properly inform prospective participants on scope of research informed consent for exempt research Includes tips, instructions, and examples for each element of. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care,. Find templates for informed consent, assent, and debriefing forms for various types of human participant research studies. This template should be used as the consent document guide for all new research studies, including parental. The consent/assent form should be in a language that is understandable to someone without a scientific background. Use these templates to properly inform prospective participants on. The consent/assent form should be in a language that is understandable to someone without a scientific background. Download the forms in doc format. The templates are updated regularly and cover. Learn about the changes to the regulations for informed consent and the irb submission. Informed consent and hipaa authorization form template v01nov2024 docx: The templates are updated regularly and cover. This template should be used as the consent document guide for all new research studies, including parental. Includes tips, instructions, and examples for each element of. Please use the microsoft readability statistics tool as needed when. The consent/assent form should be in a language that is understandable to someone without a scientific background. Template participant consent form and participant information sheet providing information about the research to participants, and gaining consent from participants before their involvement is. Please use the microsoft readability statistics tool as needed when. This resource provides points to consider and sample language for informed consent documents of research studies which plan to store and share data and/ or biospecimens. Learn how to use the new plain. It is important that principal. Download the forms in doc format. Consent document templates can be found on forms, guidance, and resources, by clicking the templates tab. Include for studies that will place research information/consent form in the participant clinical/medical record(s) (required for studies involving treatment, care, or diagnosis): Learn how to use the new plain. Use these templates to properly inform prospective participants on scope of research informed consent for exempt research Find templates for informed consent, assent, and debriefing forms for various types of human participant research studies. This template should be used as the consent document guide for all new research studies, including parental. Find templates. Includes tips, instructions, and examples for each element of. Learn about the changes to the regulations for informed consent and the irb submission. The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. Learn about the changes to the regulations for informed consent and the irb submission. Consent document templates can be found on forms, guidance, and resources, by clicking the templates tab. Learn how to use the new plain. Template participant consent form and participant information sheet providing information about the research to participants, and gaining consent from participants before their involvement. Learn about the changes to the regulations for informed consent and the irb submission. Find templates and guidelines for consent and assent forms for various types of research, including biomedical, social, behavioral, and educational. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,.. Please use the microsoft readability statistics tool as needed when. Find consent form templates and guidance for different types of research projects. Learn how to use the new plain. This template can be used by researchers to gain informed consent to conduct research that collects data from people using questionnaires, observations, interviews, diaries, focus groups,. Learn about the changes to the regulations for informed consent and the irb submission. This consent should be used to obtain permission from subjects or the parent(s) of subjects (if minors will be. Investigators are required to use the latest versions of the informed consent form templates, which have been updated to comply with the 2019. Informed consent and hipaa authorization form template v01nov2024 docx: The consent form (icf) templates provided by the irb comply with federal regulations and hipaa. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed consent forms (icf). This resource provides points to consider and sample language for informed consent documents of research studies which plan to store and share data and/ or biospecimens for. Template participant consent form and participant information sheet providing information about the research to participants, and gaining consent from participants before their involvement is. The templates are updated regularly and cover. Consent document templates can be found on forms, guidance, and resources, by clicking the templates tab. The consent/assent form should be in a language that is understandable to someone without a scientific background. Find templates for informed consent, assent, and debriefing forms for various types of human participant research studies.FREE 12+ Research Consent Form Samples & Templates
FREE 12+ Research Consent Form Samples & Templates
Research Consent Form Fill Out, Sign Online and Download PDF
FREE 12+ Research Consent Form Samples & Templates
FREE 6+ Research Consent Forms in PDF MS Word
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
FREE 12+ Research Consent Form Samples, PDF, MS Word, Google Docs
Free Research Informed Consent Form PDF Word eForms
FREE 6+ Research Consent Forms in PDF MS Word
FREE 12+ Research Consent Form Samples & Templates
Include For Studies That Will Place Research Information/Consent Form In The Participant Clinical/Medical Record(S) (Required For Studies Involving Treatment, Care, Or Diagnosis):
Download The Forms In Doc Format.
There Are Other Webpages Devoted To Providing Guidance For Writing Readable,.
Includes Tips, Instructions, And Examples For Each Element Of.
Related Post: